BillerudKorsnäs Launches SteriKraft Protect AR
![]() Print this Article | Send to Colleague
Print this Article | Send to Colleague
The standardization, packaging for terminally sterilized medical devices ISO 11607-1, 5.5 Storage & Transport, has led to more rigorous and realistic ship testing protocols which have, in turn, shown the need for higher performance medical device packaging systems. To answer this need, BillerudKorsnäs, Sweden, has developed a new polymer reinforced paper, SteriKraft® Protect AR.
SteriKraft Protect AR is designed as a heat seal coating base but will also seal peel to selected advanced peelable films without needing a coating. More challenging applications, such as large drape & gown packs, spinal needles, IV catheters etc., will benefit from the extra performance offered by SteriKraft Protect AR. As with all other papers in the BillerudKorsnäs SteriKraft range, Protect AR is fully approved under the relevant sections of EN868 and ISO 11607-1.
"The key benefit of the polymeric addition is to provide some stretch to the paper, which results in much improved tensile energy absorption (TEA). TEA gives a good measure of the durability of the paper under shipping conditions," explained Jonathan Andrews, business development director, BillerudKorsnäs Medical.
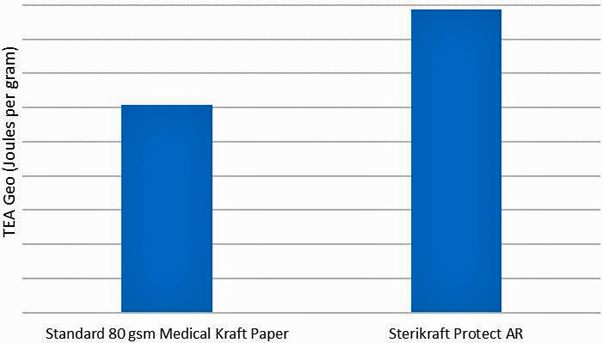
The graph above is a comparison of Geometric TEA between standard medical kraft papers and new SteriKraft Protect AR, demonstrating the significantly higher performance of the new paper.
BillerudKorsnäs SteriKraft is a medical paper that can withstand exposure to various sterilization methods and guarantees the sterility and purity of the products right up until the moment of use. The packaging will maintain its sterility for five years and is offered for all major sterilization methods. More information is available online.


